Home> Multi-stakeholder Projects> Engaging participation of consulting and informing Patient organizations and patients
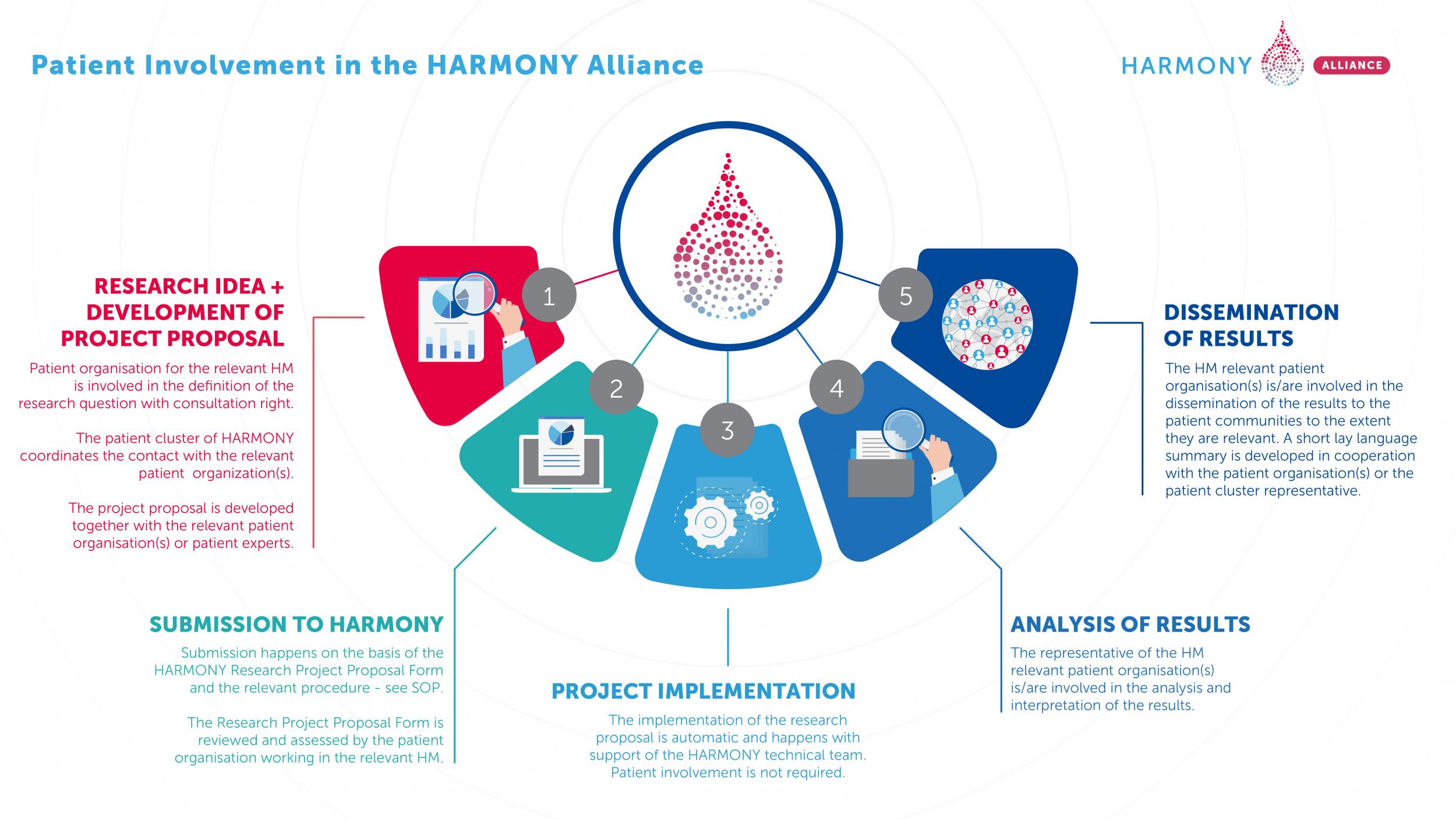
The HARMONY Alliance Patient Cluster developed a Standard Operating Procedure on how to engage participation of consulting and informing Patient Organizations and patients in Research Projects.
The importance and benefits of patient involvement across the entire health related research and development spectrum are well documented. HARMONY and HARMONY PLUS are based and built on a structure that involves patients consistently in both the project consortium and the work done by it. It is therefore logical and essentially important that patients and their organisations participating in HARMONY and HARMONY PLUS through the Patient Cluster are granted the possibility to participate in the design, submission, evaluation, interpretation, and dissemination of research projects run on the HARMONY Big Data Platform.
The Standard Operating Procedures (SOP) and Recommendations on Patient Involvement in HARMONY PLUS is a short compendium of rules and procedures to be used in relation to research projects run on the HARMONY Big Data Platform to make sure that the patient perspective is adequately and consistently represented. The HARMONY PLUS project will follow the same procedure than the one established for the submission of research project proposals in the HARMONY project (116026) regulated in the SOP for the Submission of HARMONY Research Project Proposals (see links above).
Standard Operating Procedure on how to engage participation of consulting and informing Patient Organizations and patients in Research Projects documents concerns the inclusion of patients in the development and submission of research project proposals, and the dissemination of their results. This Standard Operating Procedure and Recommendations contains a description of the benefits and recommended processes for engaging patients and patient organisations (PO) in biomedical R&D (research and development) projects and processes within the HARMONY and HARMONY PLUS projects.